Collaboratively addressing the challenges in healthcare Engineering
REACH US
Share
OUR COMPANY
Greyfalcon Healthcare (INDIA) Pvt. Ltd. is collaborating with healthcare solutions providers, research institutions and biomedical engineering teams across the world to develop engineering solutions for the problems identified in the healthcare and pharma engineering sectors. Our in-house team of experienced engineers and our manufacturing ecosystem enables us to design and develop high quality and affordable medical end equipment. We are an OEM and we provide product consulting for customized electro-mechanical designs, electronics and software engineering. Our area of focus, expertise and technical abilities are as highlighted.
Fluid mechanics and engineering
Liquids are the 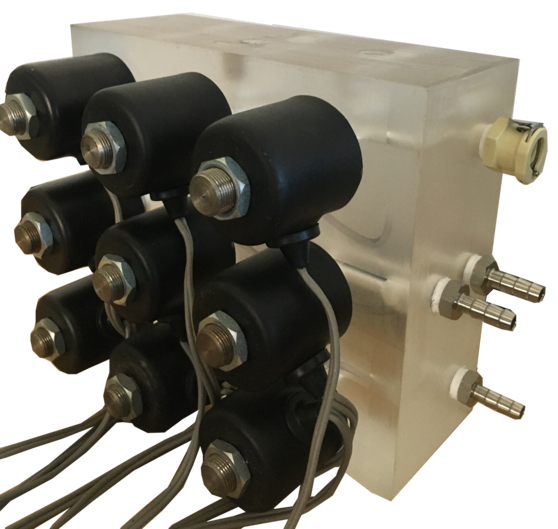 fundamental and most important medium of contact in medical equipment. The performance of the equipment depends on assessing the type of fluid, flow of fluid around and inside the equipment and engineering the accurate fluid flow solutions. Our team has extensive experience in handling any kind of fluid flow requirements of the equipment like handling the high concentrations of chemical and fumes, dealing with blood and blood clots, operating the equipment at very low or very high pressure of water from different sources, to create optimal flow distribution, efficient thermal management, minimal pressure losses, or whatever key flow performance that product requires. The team has experience in designing custom manifold blocks, using solenoid valve assemblies to control the flow of liquid, accurately measure and dispense the fluid at required flow rate, moderate the flow rate with various sensors and use various types of air and liquid pumps to transfer and pump different types of liquids.
fundamental and most important medium of contact in medical equipment. The performance of the equipment depends on assessing the type of fluid, flow of fluid around and inside the equipment and engineering the accurate fluid flow solutions. Our team has extensive experience in handling any kind of fluid flow requirements of the equipment like handling the high concentrations of chemical and fumes, dealing with blood and blood clots, operating the equipment at very low or very high pressure of water from different sources, to create optimal flow distribution, efficient thermal management, minimal pressure losses, or whatever key flow performance that product requires. The team has experience in designing custom manifold blocks, using solenoid valve assemblies to control the flow of liquid, accurately measure and dispense the fluid at required flow rate, moderate the flow rate with various sensors and use various types of air and liquid pumps to transfer and pump different types of liquids.
Sensors and control designs for medical equipments
We have successfully designed various conductivity sensor modules to measure the concentration of chemical composition in liquids and solutions. Our expertise in handling photon multipliers and sensors has helped us in designing and developing chemiluminescence modules used in high end Luminometers and IVDs. These devices are used by assay manufacturers to measure and analyze various parameters in/from human blood.
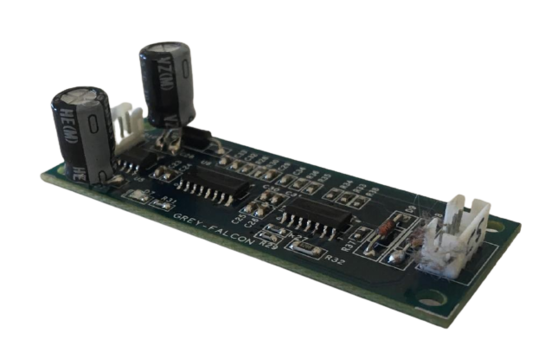

Customized interfaces and connectors
While working with fluids and chemical solvents its important to identify the medium of contact and its compatibility to the material being used to design the transfer of fluids. We have spent significant amount of engineering cycles in understanding the chemistry, reactive nature of metals and polymers to design and manufacture mating connectors for fluid transfers.
Our non-corrosive, leak and rust proof Hansen connectors are one such examples, these connectors are used in various medical applications to interface water and other high pressure liquids. We design and contract manufacture customized and standardized connectors according to the given specifications.
Medical electronics design solutions
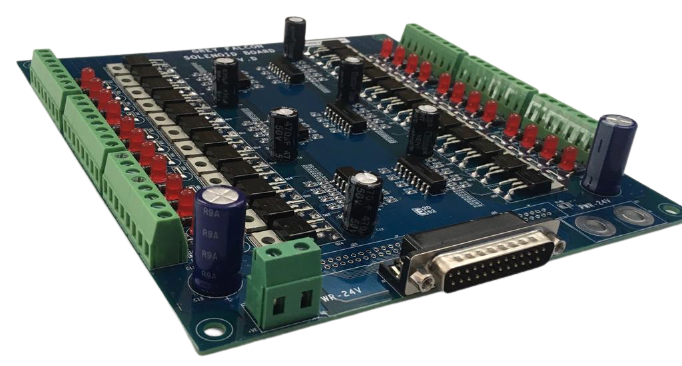 Our ISO 13485:2016 certified engineering design process is applied and strictly followed in developing and designing the electronics for medical devices. Our specialization in embedded systems, motion control, PMT, spectroscopy, microprocessors and microcontrollers and analog & mixed signal measurements helps our end customers design next generation medical equipment. We help in analog and digital circuit design for medical sensors, instrumentation, motor and fluid controls. The time to market is considerably reduced as we adopt modular engineering and circuit design, collaboratively work with specialized engineering teams and provide in-house validation and support infrastructure to meet the requirements of notified certification bodies.
Our ISO 13485:2016 certified engineering design process is applied and strictly followed in developing and designing the electronics for medical devices. Our specialization in embedded systems, motion control, PMT, spectroscopy, microprocessors and microcontrollers and analog & mixed signal measurements helps our end customers design next generation medical equipment. We help in analog and digital circuit design for medical sensors, instrumentation, motor and fluid controls. The time to market is considerably reduced as we adopt modular engineering and circuit design, collaboratively work with specialized engineering teams and provide in-house validation and support infrastructure to meet the requirements of notified certification bodies.
Systems software development and porting
Medical electronics require very simple to very complex software deployment and enablement on range of microcontrollers and multi-core architectures. Our core expertise has been in developing firmware for microcontrollers, Linux device drivers, Linux Kernel porting, OS distribution porting and Android application development for medical devices and equipment. Our extended team is capable of implementing custom ai alogrithms and solutions for devices that require high end image processing and analysis. Our ability to develop software for analog front ends, audio subsystems and display solutions have helped us develop products that cater to next generation medical devices.

FIRMWARE
Product ENGINEERING

Committed to
successful
product engineering
Our passion for healthcare end equipment manufacturing has enabled us to develop affordable, feature rich medical equipment for nephrology, ENT care and body parameter monitoring solutions. The trade mark registered products of ours are audited, qualified and registered with the ministry of health in various countries across the globe, we have cleared MDSAP audits, certified for ISO and CE.



Technology for safe and hygienic dialysis. We are one of the globally recognized and renowned brands in the field of dialyzer reprocessors and solutions. Greyfalcon Healthcare has been into manufacturing dialyzer reprocessors since the year 2016, we own the registered trademark for nephrotron, the range of reprocessors addresses various issues with the dialyzer reuse, our dialyzer reprocessors use latest in the sensor control technology to manage fluids, analog signal integration and analysis and we have developed Android based software solutions to provide technician a friendly user experience. The products are integrated with barcode scanners, polyester thermal transfer printers and Bluetooth devices.
Visit www.nephrotron.com for more information.
Download the product datasheet
toxypho is a CE certified, low power, compact, Android TM application based single tube Luminometer, designed to make the EAA analysis affordable and the easy to use design, makes the product technician friendly. The Endotoxin Activity Assay (EAATM) from Spectral Medical is the only commercially available test to measure endotoxin activity in whole blood that is cleared by the US Food and Drug Administration (FDA), licensed by Health Canada in 2003, and CE marked. Untill the EAA, there has been no reliable method to measure endotoxin accurately in the blood stream. The Luminometer can also be used for research purposes for other end user applications.
Visit www.toxypho.com for more information
Download product datasheet
